Introducing The AI Project Planner — Scope, Build, Map, Compare, and Enrich your projects with precision. Learn More →
Introducing The AI Project Planner — Scope, Build, Map, Compare, and Enrich your projects with precision. Learn More →
AI handles the grind while your people drive impact.
Optimize Efficiency. Unleash Potential.
The AI Strategic Scoper
Strategic scopes backed by competitive intelligence, not guesswork.


THE PROBLEM:
You're launching a high-stakes program. The board demands competitive positioning. Finance needs realistic budgets. Your PMO wants clear boundaries. Everyone wants it yesterday. Meanwhile, you're buried in execution while trying to research competitors, validate regulatory pathways, and build a defensible business case from scratch.
WHAT SCOPER DELIVERS:
THE OUTCOME:
Board-ready strategic scope in 48 hours, not 3 weeks. Your charter includes the competitive analysis and regulatory intelligence leadership expects. You enter funding discussions with defensible assumptions grounded in current market reality—no scrambling, no guesswork.
WHO SCOPER HELPS:
Portfolio VPs and Program Heads launching new initiatives who need investor-grade scopes that secure funding and align stakeholders—without the research burden.
Prompt:
Map strategic scenarios for antisense Lp(a)-lowering therapy commercial launch addressing potential HORIZON trial underperformance: Restricted High-Risk Coverage Gateway with specialty pharmacy networks and step therapy requirements, Delayed Broad Access with extended real-world evidence generation, Premium Pricing Strategy with value-based agreements; quantify each scenario for market penetration timeline, payer coverage restrictions, specialty pharmacy requirements, and revenue impact considering PCSK9 inhibitor precedent and Lp(a) treatment landscape dynamics.




Prompt:
Define Phase 2 advancement framework for PRMT5 inhibitor targeting MTAP-null solid tumors transitioning from dose escalation toward proof-of-concept. Establish biomarker-driven patient selection criteria and tumor response thresholds specific to synthetic lethality. Address companion diagnostic co-development and manufacturing scale-up for small molecule synthesis.

Prompt:
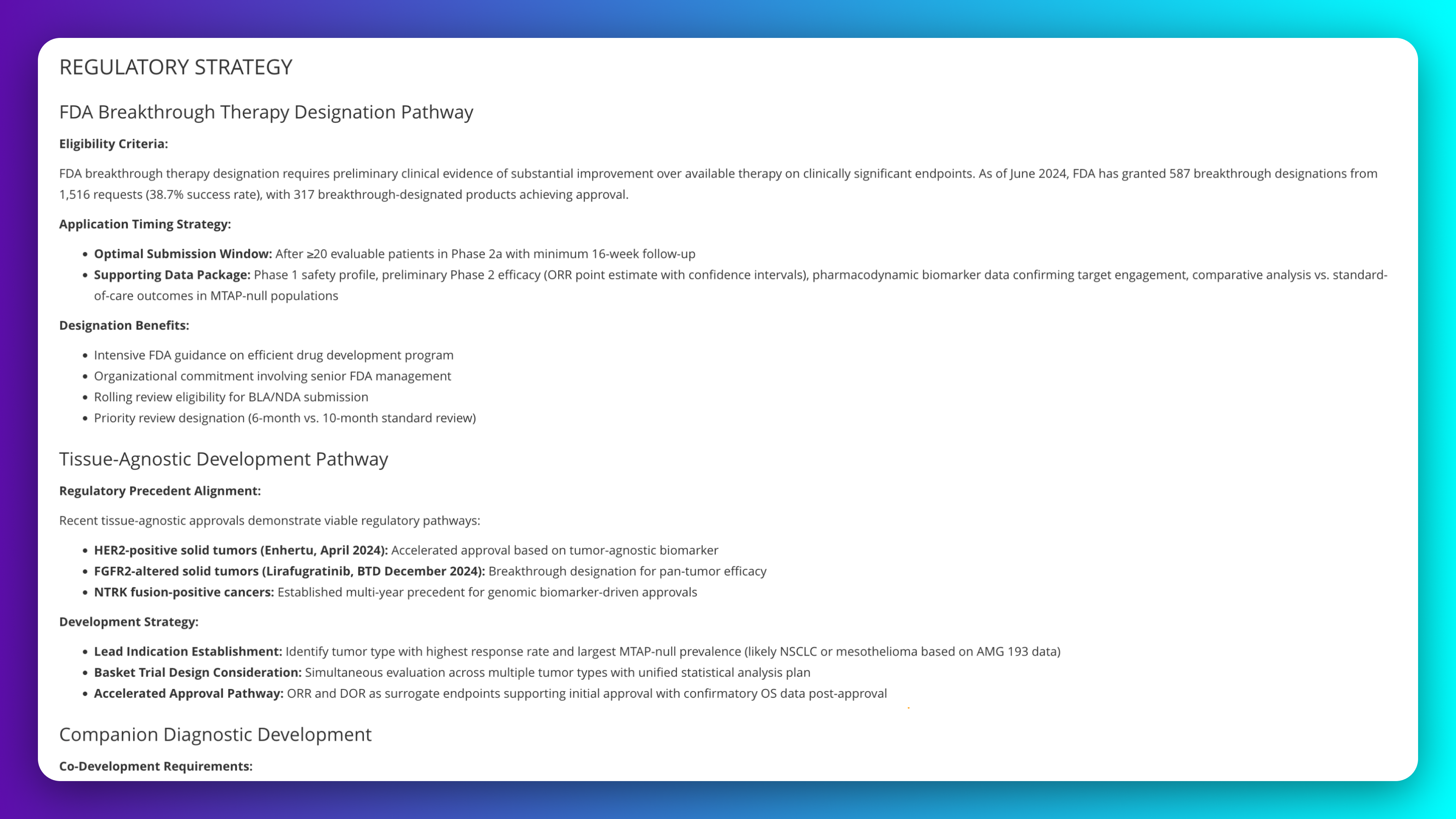
Map FDA breakthrough therapy designation pathway for tissue-agnostic development across multiple MTAP-deleted cancer types. Align precision oncology strategy with recent FDA approval precedents for biomarker-driven indications. Identify optimal submission timing, supporting data package requirements, and designation benefits for accelerated development timelines.
Prompt:
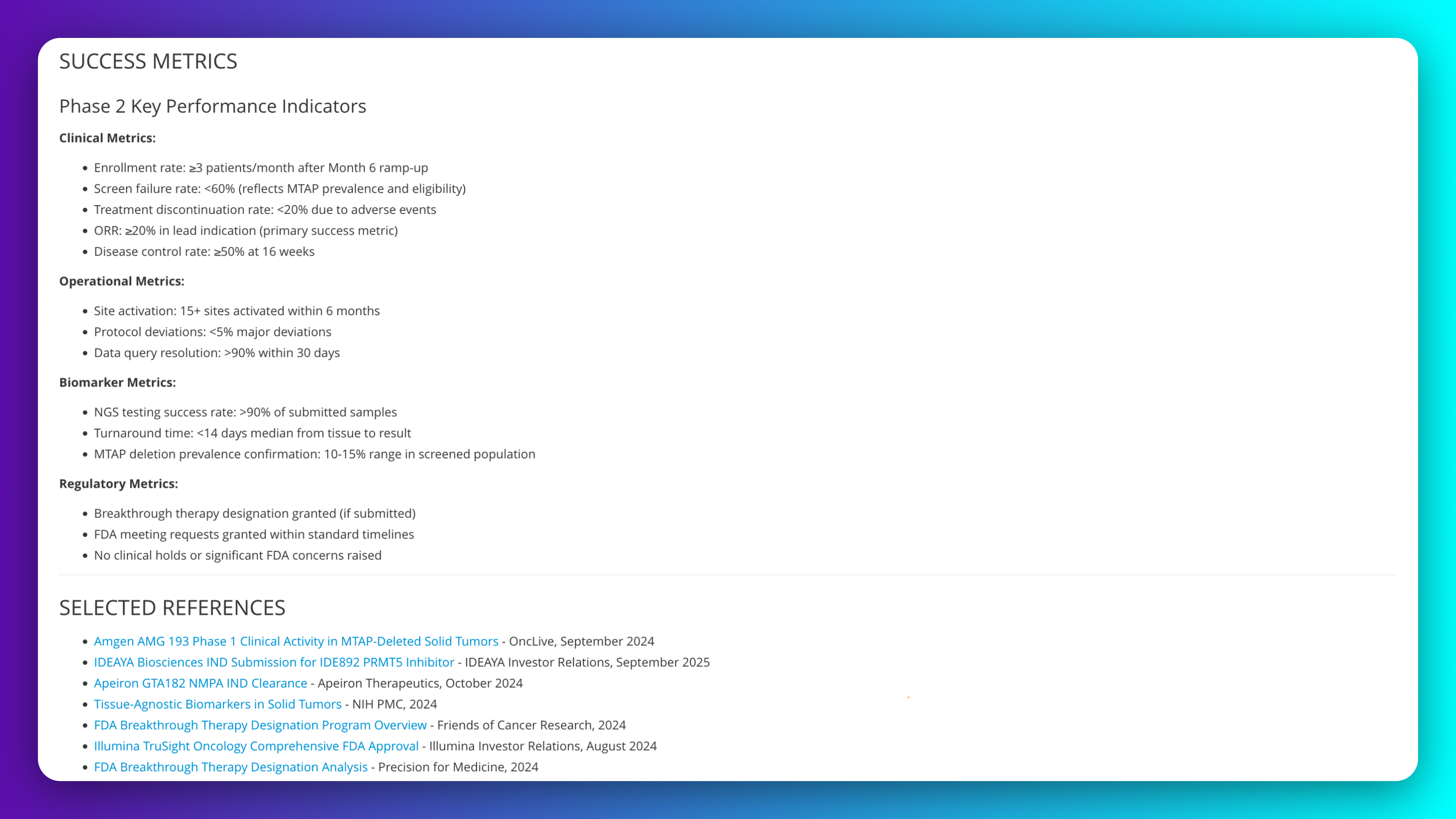
Establish measurable success criteria for Phase 2 PRMT5 inhibitor program in MTAP-null solid tumors with clinical efficacy thresholds, operational performance metrics, and regulatory milestone gates. Include NGS testing benchmarks for MTAP deletion screening and go/no-go decision criteria grounded in comparable synthetic lethality program precedents.
Use Cases
First-in-Class Antisense Therapy for Lp(a) Reduction
Builder | Industry | This is a function
Modular Biosecure Hospital Pod: Strategic Development and Global Deployment
Builder | Industry | This is a function
Global Real-Time IoT + Cold-Chain Monitoring AI Logistics
Builder | Industry | This is a function
AI-Driven Real-Time Utilization Management Decision Engine
Builder | Industry | This is a function
Next-Gen Spatial Multiomics Platform Development Strategy
Builder | Industry | This is a function
Smart Wound Dressing with Biosensors + Paced Drug Release
Builder | Industry | This is a function
Next-Gen Bidirectional Neural Interface BMI: Launch-Readiness
Builder | Industry | This is a function
PCSK9 Base Editing PreClinical Therapeutic Development
Builder | Industry | This is a function
MRI Diagnostic SaMD AI Tool: Regulatory Development
Builder | Industry | This is a function
Scalable Hospital-at-Home for Advanced Acute Care Delivery
Builder | Industry | This is a function
HER2×PD-L1×VEGF Trispecific Antibody Development Program
Builder | Industry | This is a function
Unipr is built on trust, privacy, and enterprise-grade compliance. We never train our models on your data.






