The Coordination Fallacy: Two Realities in Drug Development
The artifacts of coordination prevent the coordination they promise.
The Coordination Fallacy
We believe we're coordinated because we have the artifacts of coordination: plans, governance meetings, integrated decks. But the artifacts themselves prevent the coordination they promise. They produce one reality for leadership and another for execution. By the time the consolidated deck reaches decision-makers, the territory has already moved.
This is the coordination fallacy: mistaking the presence of coordination artifacts for the presence of coordination itself.
After thirteen years building coordination systems for pharmaceutical organizations, I've watched this fallacy play out across companies of every size. The plan exists. The reality exists. They occupy separate universes, and the gap between them is where medicines stall and patients wait.
The Proof Point
In December 2023, Bluebird Bio celebrated FDA approval of Lyfgenia, a gene therapy for sickle cell disease. The plan had worked: years of clinical development, a successful regulatory submission, a groundbreaking therapy for a patient population desperately waiting. The artifact was complete.
Two years later, the company no longer exists.
On January 12, 2026, a company now called Genetix Biotherapeutics announced an emergency expansion of its manufacturing partnership with Minaris. The reason? "Although we are the market leader," CEO David Meek acknowledged, "the vast majority of patients have not yet received treatment."
The approval plan had succeeded. The execution reality had failed. And the gap between them proved fatal to the original company's identity.
This isn't a story about manufacturing complexity or gene therapy challenges. It's a story about what happens when the reality shown to leadership diverges from the reality guiding execution, and no one has the visibility to see it coming until it's too late.
The Information Latency Problem
When we talk about "planning artifacts" in drug development, we mean the objects everyone recognizes: the Excel files tracking timelines, resources, and dependencies that each function maintains separately. The PowerPoint decks assembled for leadership reviews, polished and confident, increasingly disconnected from ground truth. The project files in MS Project, Smartsheet, or Planisware that each function maintains in its own format, on its own cadence, with its own assumptions. The status reports that roll up from teams, sanitized through layers of reporting before reaching leadership.
The pattern is always the same. Clinical maintains its version. CMC maintains its version. Regulatory maintains its version. Commercial maintains its version. Someone, or many someones, manually assembles the "integrated view." By the time it's reconciled across functions, each function's reality has moved. The consolidated artifact is a snapshot of a moment that no longer exists.
This isn't a planning problem. Organizations don't lack planning discipline or sophisticated tools. What they have is an information latency problem: the structural gap between reality changing and the plan reflecting it.
A recent McKinsey survey found that only 20% of biopharma leaders believe their operating model enables timely cross-functional decision-making. Turn that around: 80% of leaders acknowledge their operating model does NOT enable timely cross-functional decisions.
They know. They just can't see clearly enough to fix it.
As I explored in Four Companies Wearing One Logo, pharmaceutical organizations weren't designed to operate as integrated systems. They evolved function by function, acquisition by acquisition. Each function optimized for its own excellence. What we're now discovering is that the planning systems followed the same path, creating not one organizational reality but two: the one leadership reviews, and the one teams navigate daily.
Six Months to Obsolescence
How does a plan become fiction? Not through dishonesty, but through process.
Consider what McKinsey documented in their March 2025 analysis of pharmaceutical planning processes: Financial and brand planning at one organization "took more than six months and required input from nearly everyone, from brand teams to the CEO."
Six to eight months. That's the cycle time for the governance artifact.
By definition, a six-month planning cycle produces a document that reflects conditions from six months ago. The plan is approved based on assumptions that have already changed.
The moment of 'approval' is also the moment of obsolescence.
But the divergence doesn't stop there. Once approved, the plan enters maintenance mode: updated monthly, sometimes quarterly, for governance presentations. Meanwhile, execution reality changes daily. A trial enrollment slows. A manufacturing campaign shifts. A regulatory question emerges. A supplier constraint surfaces. The people closest to the work know immediately. The governance artifact won't reflect it for weeks or months.
The result is two parallel tracks: leadership reviewing one reality in monthly steering committees and quarterly portfolio reviews, while execution teams navigate a different reality through spreadsheets, emails, and hallway conversations. The gap widens with every passing week.
Executives believe they're steering; they're actually reacting to hindsight.
This isn't a failure of effort. It's a failure of architecture. The planning systems were designed for presentation, not for work.
Why Realities Diverge
Four forces produce this split. They're not independent problems to solve separately. They're reinforcing dynamics that create a single outcome: leadership and execution inhabit different versions of the same program.
Plans serve different masters. Leadership needs confidence and predictability. Boards want dates. Investors want timelines. The governance artifact must project certainty. Execution needs flexibility and honesty. Real work is probabilistic. Timelines depend on variables not yet known. The execution reality must accommodate uncertainty. These demands are fundamentally incompatible. No single artifact can satisfy both, so organizations maintain two: one for presentation, one for work.
Artifacts enforce fragmentation. Each function maintains its own plan in its own format on its own cadence. Clinical uses one timeline structure. CMC uses another. Commercial uses a third. Enterprise data shows that only 28% of applications in large organizations are integrated. That means 72% are siloed, their data inaccessible to other systems without manual extraction and reconciliation. The "integrated plan" isn't a single source of truth. It's a manually-assembled composite that begins diverging from its component sources the moment it's created.
Worse, functions evolved different languages for the same milestones. Your clinical team calls it "Database Lock." Your regulatory team calls it "Data Cutoff." Your manufacturing team calls it "Batch Release Trigger." They all mean the same thing. But the systems that hold these terms have no idea they're synonyms. Field mapping is brittle, manual, and breaks whenever source systems change. Integration moves data, but meaning gets lost in translation.
Precision demands create planning fiction. Systems want a date field. Real work is probabilistic. "Maybe Q3 if three things go right" isn't an acceptable entry in a project management system. So people enter a date, knowing it's a guess, knowing it will change, knowing that the system will record it as a commitment. Research on clinical trials found that 55% of trials change their primary outcomes during the course of the study. The plan captured at trial start diverges from execution reality in more than half of cases, often without timely updates to the official record.
The plan becomes a compliance artifact. Updated for governance meetings, not for decisions. The "real plan," the one that actually guides work, lives somewhere else: in spreadsheets on individual laptops, in email threads, in tribal knowledge held by the people closest to the work. The governance artifact exists to be presented, not to be used. It's a document that checks a box, not a tool that enables coordination.
These four forces don't produce four separate problems. They produce one: the divergence of what leadership sees from what teams experience.
The 40% Tax
What do alternate realities cost? The evidence points to a substantial overhead that most organizations simply accept as the cost of doing business.
Consider protocol amendments, the most visible evidence of plans that couldn't survive contact with reality. Tufts Center data shows that 76% of clinical trials require protocol amendments. The plan was created, approved, and initiated. Then reality diverged, requiring formal correction.
Not all amendments are avoidable. Science is uncertain. Conditions change. But 45% of protocol amendments trace to causes that could have been prevented with better upstream planning: eligibility criteria that didn't match the patient population, endpoints that didn't align with regulatory expectations, logistics that weren't coordinated across sites.
Each amendment costs $141,000 to $535,000 in direct expenses. Each takes an average of 260 days to implement. In a world that changes daily, a 260-day correction cycle isn't a lag. It's an autopsy.
Protocol amendments are the audit trail of alternate realities. They document the gap between the plan that was approved and the execution that was required.
The broader cost is harder to quantify but equally real. McKinsey's January 2025 analysis found that companies can cut time to first-in-human by 40% or more through process improvements alone. Not better science. Not new technology. Process improvements.
One improvement they identified: "Organizations that effectively redesign governance to improve speed often find success by consistently adhering to minimum-viable requirements for each stage or simplifying the templates for milestone presentations."
Simplifying templates for milestone presentations. That's McKinsey's polite way of saying: stop producing elaborate governance artifacts that don't guide execution. The 40% time reduction potential suggests that current planning processes add substantial overhead: the tax of maintaining two realities instead of one.
Decisions made on fiction produce fictional outcomes. The 'surprise' delay at Phase III was visible months earlier, just not to the people making decisions.
The industry knows this is unsustainable. In that same McKinsey survey, 37 of 50 leaders expect simplification efforts in the next 12 months. 32 believe they need a "significantly different operating model." They recognize the problem. What they lack is a path through it.
What "Better" Actually Looks Like
The question isn't whether the two-realities problem is real. The evidence is overwhelming. The question is whether it's fixable.
Evidence suggests it is, but the solutions demonstrated to date have required a significant trade-off: they work by eliminating the coordination problem rather than solving it.
Consider what Eli Lilly's Chorus unit achieved. A decade-long experiment in alternative development, published in Nature Reviews Drug Discovery, showed that Chorus reached proof-of-concept in 28 months at $6.3 million, compared to the traditional 48 months and $42 million. That's a 40% reduction in time and an 85% reduction in cost.
How did they do it? Not through better planning software or more rigorous governance. They did it by collapsing the coordination problem entirely.
Chorus operated with roughly 40 people, co-located under one roof, reporting through a single managing director. The paper describes their approach: "The flat organization structure reporting up through a single line of management eliminates the function-team matrix dynamic, which slows decision-making." They minimized governance oversight. They used a two-person team for each asset instead of the traditional cross-functional matrix. They operated on what they called "phase-appropriate policies," meaning: only the governance artifacts that were absolutely necessary, nothing more.
In other words, Chorus collapsed the two realities into one by putting everyone in the same room.
This works brilliantly for 15-17 projects with 40 people. The governance artifact and the execution reality were the same thing because the same people created both. When the person creating the plan sits next to the person executing it, when both report to the same manager, when all 40 people can gather around a conference table, there's no gap for divergence.
This is how a single-celled organism works. All its functions exist in one place. When conditions change, the whole organism knows immediately. There's no coordination problem because there's nothing to coordinate between.
Chorus was essentially a single-celled development organization.
But enterprise pharma isn't a paramecium. It's a complex multicellular organism. You can't run 300 clinical trial sites across 19 countries with a two-person team. You can't coordinate 6 global research centers and 10 manufacturing units through hallway conversations. You can't manage a portfolio of 50+ programs with 40 co-located people. Enterprise pharmaceutical development requires thousands of people, dozens of systems, and hundreds of handoffs across functions that will never share a single room.
From Two Realities to One
The goal isn't more planning. It's eliminating the lag between reality changing and leadership knowing.
And the answer isn't to collapse a multicellular organism into a single cell. That's not possible at enterprise scale, and it's not desirable. Specialization exists for good reasons. The heart specializes in pumping blood. The lungs specialize in gas exchange. Clinical specializes in trials. CMC specializes in manufacturing. Each function has earned its expertise, its tools, its artifacts. The goal isn't to collapse them into one room or force them to use identical planning systems.
But here's what a healthy body has that most pharmaceutical organizations lack: coordination infrastructure. The nervous system carries signals between organs in real time. When you start running, your brain doesn't wait for a quarterly review to tell your heart to speed up.
Enterprise pharma has the organs. What's missing is the nervous system between them.
This requires a critical distinction: connected systems are not coordinated organizations. Most enterprises have invested heavily in integration, moving data between systems, and in data platforms, storing and analyzing information. But integration platforms are pipes. Sophisticated pipes, but pipes. They move data. They don't understand it.
Your systems are connected. Your organization is not.
Two Paths to Coordination at Scale
Beyond Chorus-style coordination, where small teams keep artifacts aligned through constant conversation, enterprise scale offers two architectural paths. Both have been tested. Both produce results. They differ in ambition, timeline, and what they require organizations to change.
Path 1: Build unified from the ground up.
Every artifact-generating application designed from scratch to stay coordinated, part of a unified technology platform where all tools share the same underlying data model. Novartis calls this approach "Lean Digital Core." Their strategy involves building a comprehensive data ecosystem where cross-functional teams can access integrated data from multiple sources through a unified stack.
The results, where achieved, are significant. GSK's Onyx platform, designed to "build a comprehensive data and machine learning ecosystem," has enabled their scientists to work from shared data rather than reconciled snapshots. Companies that complete this transformation report 50% reductions in planning cycle time and 20% improvements in product delivery speed.
But this path takes years. It requires replacing tools that functions have used for decades. It demands organization-wide alignment on a single data model before anyone sees benefit. Only 3 of 50 pharmaceutical leaders surveyed by McKinsey believe they've solved the data-to-decision bottleneck. The technical elegance is real. So is the implementation difficulty.
Path 2: Coordinate existing artifacts through semantic understanding.
Different tools, different formats, different owners, but connected through a coordination layer that understands how artifacts relate. Not just integration that moves data, but coordination that understands meaning.
This is the approach that CDISC and standards bodies have pioneered with the BRIDG model: a semantic framework that maps concepts across clinical, regulatory, and manufacturing domains. When "Database Lock" in the clinical system occurs, the coordination layer knows this affects "Data Cutoff" in regulatory and "Batch Release Trigger" in manufacturing, even though they use different terms in different tools.
The evidence suggests this works. One pharma company implementing semantic coordination cut their biosample management cycle time by 40% while improving data quality and regulatory compliance. The key: unified understanding of meaning across artifacts, without forcing everyone onto a single platform.
This path preserves functional autonomy while enabling cross-functional alignment. Clinical keeps its systems. CMC keeps its systems. Regulatory keeps its systems. But when a milestone shifts in one artifact, dependent milestones in other artifacts surface automatically. The artifacts stay specialized. The coordination happens between them.
The design philosophy shift
Both paths require the same fundamental reorientation:
From manual reconciliation to real-time signals. Today, someone manually compiles the master tracker from 15+ functional spreadsheets and tools. That's like having a human messenger run between your heart and lungs to report on oxygen levels. The signal should travel automatically, continuously, so the picture stays current without heroic manual effort.
From governance theater to decision support. The purpose of planning artifacts should be to enable decisions, not to demonstrate that planning happened. Minimum-viable governance means leadership sees what they need to decide, in time to decide it.
The industry doesn't need to coordinate everything at once. You can't transform a global organization overnight. But you can start with one medicine. One program. Get all the artifacts for that program (Clinical, CMC, Regulatory, Commercial) aligned and coordinated. Then grow from there.
Beyond connection lies coordination. That's the imperative. Specialized functions with their own artifacts, connected by coordination infrastructure that surfaces conflicts before they cascade, that lets leadership see execution reality in real time, that enables the whole organism to respond as one.
The Patient Who Is Still Waiting
Bluebird Bio had a plan. They had execution teams working toward it. What they lacked was the coordination infrastructure that would have surfaced the manufacturing gap before it became existential. They lacked the visibility that would have let leadership govern from reality rather than from a governance artifact documenting a past already obsolete.
The artifact problem is solvable. The coordination infrastructure can be built. One program at a time.
Until we do, patients like those still waiting for Lyfgenia will continue waiting.
References
- McKinsey & Company. "Strengthening the R&D Operating Model for Pharmaceutical Companies." McKinsey Life Sciences Insights, 2024. mckinsey.com
- McKinsey & Company. "Simplification for Success: Rewiring the Biopharma Operating Model." McKinsey Life Sciences Insights, March 2025. mckinsey.com
- MuleSoft. "2024 Connectivity Benchmark Report." MuleSoft Research, 2024. mulesoft.com
- Calvert M, et al. "Outcome Reporting in Clinical Trials: Analysis of ClinicalTrials.gov Records." PMC/National Institutes of Health, 2023. pmc.ncbi.nlm.nih.gov
- Tufts Center for the Study of Drug Development. "Protocol Amendment Analysis and Cost Impact Studies." CSDD Impact Reports, 2024. csdd.tufts.edu
- McKinsey & Company. "Operational Excellence in Biopharma Research and Early Development." McKinsey Life Sciences Insights, January 2025. mckinsey.com
- Owens PK, et al. "A Decade of Innovation in Pharmaceutical R&D: The Chorus Model." Nature Reviews Drug Discovery, December 2014. nature.com
- McKinsey & Company. "Rewired: Pharma Companies Will Win in the Digital Age." McKinsey Life Sciences Insights, 2023. mckinsey.com
- Clinical Data Interchange Standards Consortium. "BRIDG Model: Biomedical Research Integrated Domain Group." CDISC Standards, 2024. cdisc.org
- Grisel L. "Pharma's Simplification Imperative: From McKinsey's Vision to Implementation Reality." Medium, 2024. medium.com
Further Reading
From Unipr Insights:
- Four Companies Wearing One Logo: The Architecture of Disconnection — Why pharmaceutical organizations operate as four separate companies sharing a single logo, and what that means for cross-functional coordination
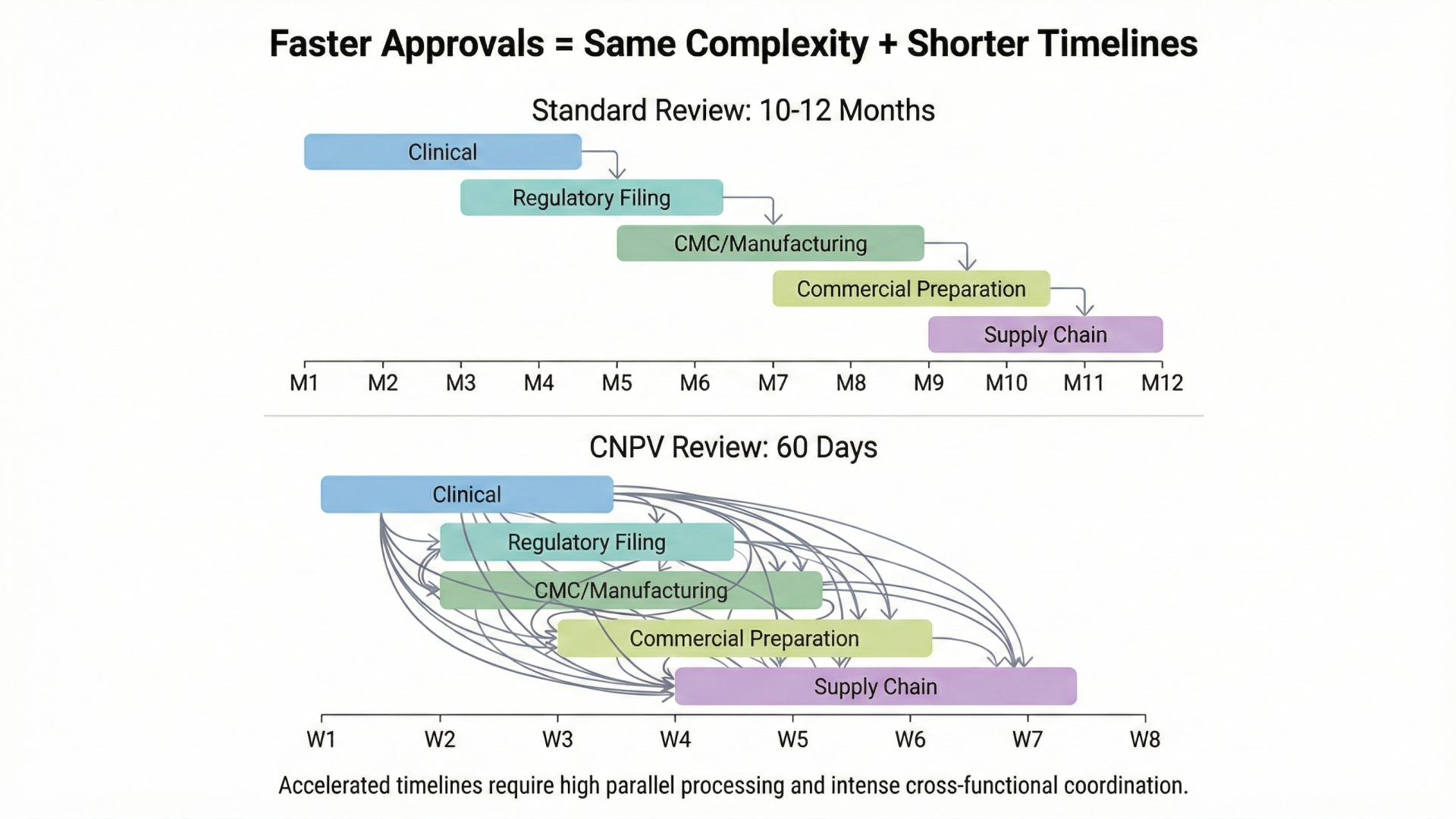
- The Buffer Illusion: When Faster Approvals Expose Slower Organizations — How accelerated regulatory timelines are exposing coordination gaps that were previously hidden by slower FDA reviews
- Why Connecting Data Isn't Enough: The Coordination Imperative — The difference between connected systems and coordinated operations, and why integration alone doesn't solve the problem
- Reimagining Planning in Life Sciences with AI Agents — How AI-powered coordination can bridge the gap between governance artifacts and execution reality
Compliance
Unipr is built on trust, privacy, and enterprise-grade compliance. We never train our models on your data.






Start Building Today
Log in or create a free account to scope, build, map, compare, and enrich your projects with Planner.