The Buffer Illusion: When Faster Approvals Expose Slower Organizations
CNPV didn't create the coordination problem. It removed the buffer that made the problem tolerable. Speed without coordination isn't an advantage. It's an exposure.
In July 2025, Replimune received a Complete Response Letter for RP1, their oncolytic immunotherapy for advanced melanoma. The rejection surprised the industry. The pre-approval inspection had gone smoothly. The clinical data was strong. By conventional metrics, the program was on track.
The rejection cited manufacturing concerns. The issues emerged not from inspection failures but from the gap between clinical-scale production and commercial-scale readiness. Replimune isn't alone. Capricor received a similar letter the same month for CAP-1002, their Duchenne muscular dystrophy therapy. Again, the issue wasn't safety or efficacy. It was analytical methods and manufacturing processes that couldn't bridge the distance between what worked in trials and what would work at scale.
These aren't stories of scientific failure. They're stories of coordination failure.
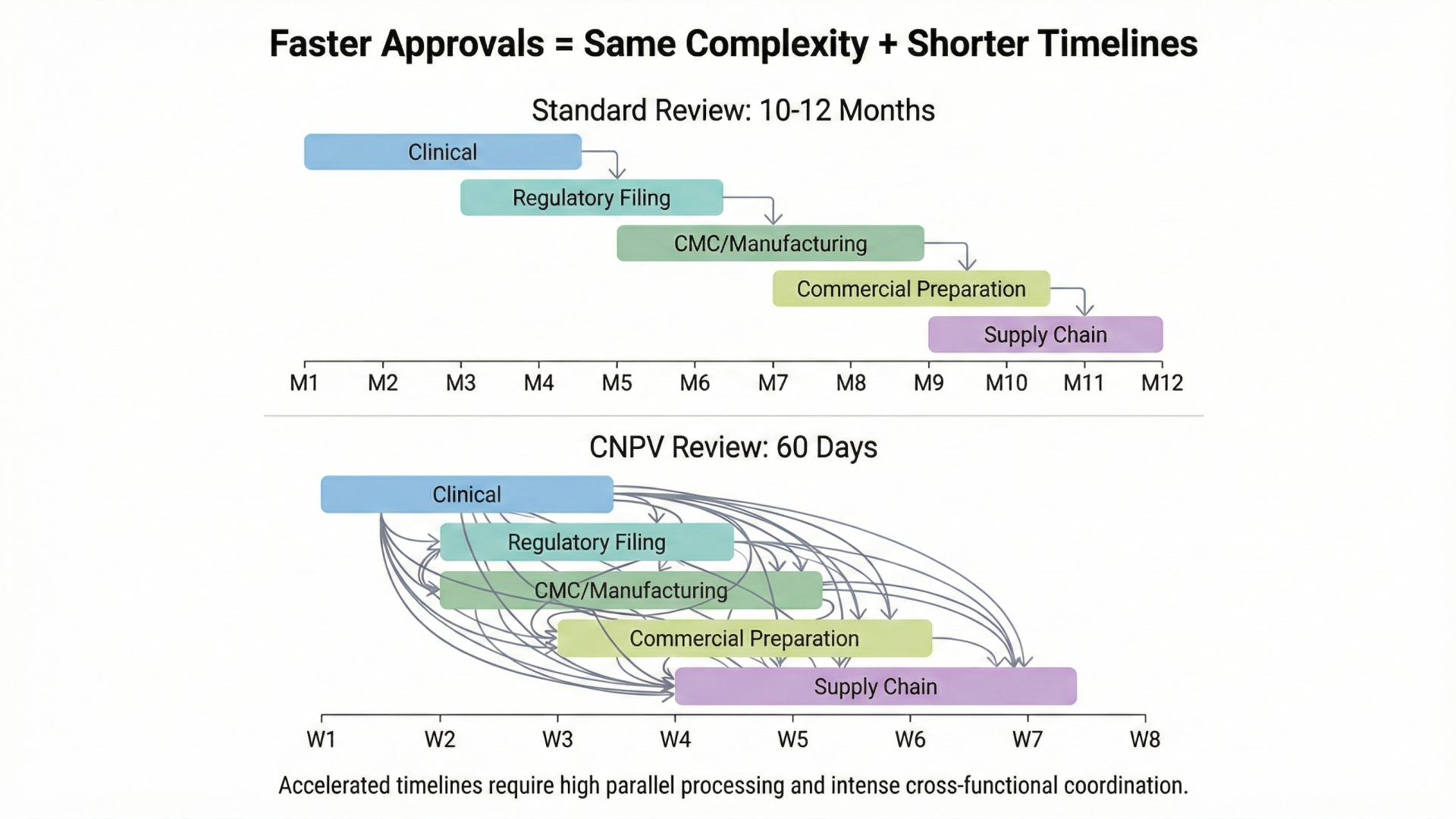
The Buffer Nobody Knew They Had
For decades, pharmaceutical development operated on a sequential model. Clinical development completes, then regulatory submission begins, then manufacturing scales, then commercial launch prepares. Each function hands off to the next. The 10-12 month FDA review period provided space (often unconscious space) for functions to catch up, align, and resolve integration issues that hadn't been addressed during development.
Manufacturing teams used review time to complete tech transfer. Commercial teams finalized pricing strategies. Regulatory teams resolved labeling questions. Quality systems addressed gaps identified during pre-approval inspections. The buffer was invisible because it was built into the system's rhythm. Everyone assumed the time belonged to FDA. In practice, much of it belonged to coordination.
FDA's Commissioner's National Priority Voucher program eliminates this buffer entirely.
Launched in March 2025, CNPV offers qualifying applications a 60-day review timeline, compressing what typically takes 10-12 months into less than two. Eighteen vouchers have been awarded. The first approval, Sandoz's Augmentin XR for antibiotic shortage, moved from submission to market in weeks.
The program requires CMC submissions 60 days before the application. This is not a nice-to-have but an eligibility criterion. Manufacturing must be commercially ready before regulatory review begins, not during it. Quality systems must be inspection-ready with no time to fix gaps. Pricing and affordability commitments must be finalized, not negotiated post-approval.
Here's the uncomfortable truth: if pharmaceutical organizations don't transform how they coordinate, faster regulatory review will widen the gap between approval and patient access, not shrink it.
Regulatory will declare victory sooner. Everything else will still be playing catch-up.
Sequential Architecture in a Parallel World
The fundamental mismatch is architectural.
Most pharmaceutical organizations evolved function by function, regulation by regulation, acquisition by acquisition. Clinical reports to a Chief Medical Officer. Regulatory reports to a Chief Regulatory Officer. Manufacturing reports to operations leadership. Commercial reports to revenue leadership. Each function optimizes for its own metrics: clinical for trial quality, regulatory for approval probability, manufacturing for batch success rate, commercial for launch revenue.
These metrics don't inherently conflict, but they don't naturally converge either. When timelines are generous, the lack of convergence is invisible. Programs move from function to function, and eventually medicines reach patients.
The obvious response is that companies will simply prepare better. But sequential coordination isn't a conscious choice. It's an organizational architecture. Functions optimize for their own metrics because that's how they're measured, how careers advance, and how budgets get allocated. Changing measurement systems requires leadership courage that most organizations lack. The same dynamics that created the coordination problem will resist solving it.
Last week, I described pharmaceutical organizations as "four companies wearing one logo." CNPV reveals the temporal dimension of that fragmentation. Each function operates on its own clock. In the sequential model, these clocks don't need to synchronize. They run in series. Clinical finishes, then regulatory starts, then manufacturing completes, then commercial launches.
CNPV demands parallel execution. All four clocks must reach completion simultaneously.
Consider what this means in practice. Clinical to manufacturing: CMC submissions must be filed 60 days before the application, meaning manufacturing must be commercially ready before FDA begins review. Clinical to pricing: affordability commitments are required for voucher consideration, meaning pricing decisions must precede regulatory submission. Regulatory to coverage: as Jones Day analysis observed, fast regulatory approval doesn't guarantee fast coverage. Payer engagement must parallel regulatory submission, not follow it. R&D to commercial: sales force training, KOL engagement, and distribution agreements should be substantially complete before voucher consideration.
Each of these dependencies existed before CNPV. The difference is that traditional timelines provided slack. CNPV removes the slack and reveals whether organizations have built true coordination or were simply relying on buffer.
The 74% Problem
The manufacturing dimension deserves particular attention because the data is stark.
From 2020 to 2024, 74% of FDA Complete Response Letters cited CMC, quality, or manufacturing deficiencies as the primary reason for non-approval. Not safety concerns. Not efficacy failures. Manufacturing and quality gaps.
This represents a dramatic shift. From 2000 to 2012, only 15% of CRLs cited CMC issues. The five-fold increase reflects both the growing complexity of modern therapeutics (biologics, cell therapies, gene therapies) and the growing gap between clinical-scale and commercial-scale manufacturing requirements.
The pattern is especially pronounced in cell and gene therapy, where 50-75% of rejections trace to CMC rather than safety or efficacy. Potency assay validation. Tech transfer protocols. Comparability between clinical and commercial materials. These aren't exotic failure modes. They're predictable consequences of manufacturing complexity addressed too late in development.
The Replimune and Capricor rejections fit this pattern. So does Gilead's Sunlenca (glass vial incompatibility), Eli Lilly's Ebglyss (comparability concerns between clinical and commercial materials), and Portola's AndexXa (observations at inspection that weren't "critical" until they were).
CNPV didn't create the coordination problem. It removed the buffer that made the problem tolerable.
When review periods compress, there is no time for CMC gaps to be resolved. What had been manageable integration challenges become disqualifying deficiencies.
What Parallel Coordination Actually Looks Like
If sequential architecture is the disease, what does the cure look like?
Gilead's Livdelzi launch offers a benchmark. When FDA granted accelerated approval for seladelpar in August 2024, Gilead wrote its first commercial prescription within hours. Not days. Hours. The company had built what they called a "Launch Excellence Team," a cross-functional integration that ensured 100% commercial readiness at the moment of approval.
That's not faster handoffs between functions. That's eliminating handoffs entirely by treating the path from clinical data to patient access as a single integrated program.
The organizations that succeed in the CNPV era won't be the ones with the best science alone. They'll be the ones where cross-functional coordination is built into the operating model, not bolted on as an afterthought when regulatory files. Manufacturing readiness achieved before submission. Pricing committed before regulatory engagement. Commercial strategy aligned before approval. Payer negotiations advanced before launch.
This changes competitive dynamics fundamentally. Traditional model: first-mover advantage goes to whoever gets PDUFA approval first. CNPV model: first-mover advantage goes to whoever earns the voucher AND can actually launch. A company with a voucher and operational readiness beats a competitor with better science and organizational dysfunction.
This may actually favor smaller, more agile biotechs. Large pharma has resources but also legacy infrastructure: sequential habits encoded in governance structures, committee calendars, and functional scorecards built over decades. A 200-person biotech building its operating model from scratch can design for parallel coordination. A 50,000-person pharma company must overcome organizational archaeology. Resources matter, but so does organizational debt. CNPV rewards coordination capability, which is orthogonal to company size.
For blockbuster drugs generating $1-15 million per day during peak sales, the 63-day average gap between approval and first prescription isn't just inconvenient. It's expensive. And if CNPV accelerates approval but organizations aren't ready to launch, the gap may extend beyond 63 days as manufacturing, coverage, and distribution catch up.
Speed is no longer something companies can buy. It's something they must build.
Three Companies That Will Prove the Thesis
One of them faces this test twice.
CNPV has been active for less than a year. Of the eighteen vouchers awarded, most have gone to supplemental indications for already-marketed products, domestic manufacturing for existing drugs, or programs that had already navigated the coordination gauntlet before receiving the voucher. The real test will come from novel drug programs still in development.
Three companies stand out.
Regeneron's DB-OTO is a gene therapy for hearing loss, still in Phase I/II. The voucher was awarded prospectively, before FDA submission. Regeneron must now coordinate gene therapy manufacturing at commercial scale, a domain where CMC complexity has historically caused the majority of rejections. Can they build parallel coordination across clinical, regulatory, and manufacturing in real time?
Revolution Medicines' Daraxonrasib is a pancreatic cancer treatment currently in Phase III. When the voucher was awarded, the company noted it would not change their development timelines. The question is whether coordination will catch up to clinical progress, or whether the gap between trial completion and commercial readiness will persist.
Merck faces the most telling test of all: two novel drugs navigating CNPV simultaneously.
Enlicitide decanoate is an oral PCSK9 inhibitor with completed Phase III trials. Sacituzumab tirumotecan is an antibody-drug conjugate for solid tumors, licensed from Kelun-Biotech and already approved in China. Both programs received vouchers within the same December 2025 announcement. Both will require coordinated execution across clinical, regulatory, manufacturing, pricing, and commercial functions. Both will stress the same organizational infrastructure at the same time.
If any company should be able to demonstrate parallel coordination at scale, it's Merck. They have the resources, the infrastructure, and decades of experience bringing complex therapeutics to market. But resources and experience don't automatically translate into coordination. The sequential habits of big pharma run deep. Merck's dual test will reveal whether organizational capability actually exists, or whether the appearance of coordination has simply been the byproduct of generous timelines.
We will track not just submission-to-approval timelines for these programs, but approval-to-first-prescription, approval-to-coverage, approval-to-patient-access. Because CNPV success isn't measured at regulatory clearance. It's measured when patients actually receive the medicine.
If these programs achieve same-day or same-week launches, CNPV will have demonstrated that coordination capability can be built. If approval-to-access gaps persist or widen, CNPV will have proven that faster regulatory review, without operational transformation, simply moves the bottleneck downstream.
What 60-Day Readiness Requires
The organizational implications extend beyond timeline compression.
Manufacturing can no longer be treated as technical execution downstream of clinical development. Manufacturing readiness determines voucher eligibility. Quality system maturity determines approval probability. Domestic manufacturing capability determines national priority alignment. CMC leadership belongs in strategic planning conversations, not just technical reviews.
Pricing becomes a development decision, not a post-launch negotiation. Four CNPV recipients committed to Most Favored Nation pricing as condition of voucher consideration. Affordability commitments are now regulatory requirements. Pricing strategy must be finalized during development, with cross-functional alignment on market positioning before regulatory submission.
Integrated planning must begin at Phase III design. Manufacturing scale-up, regulatory strategy, commercial positioning, and payer engagement must start during late-phase clinical development, not after clinical results are known. The decisions made in protocol design constrain every downstream function. If those decisions are made without cross-functional input, coordination gaps are designed into the program from the start.
Real-time decision-making capability becomes essential. When FDA can award vouchers within days of Phase III results, organizations must respond within hours, not weeks. This requires pre-positioned decision authority, pre-aligned cross-functional leadership, and pre-approved response frameworks. Traditional committee structures that convene monthly cannot move at this speed.
These aren't incremental adjustments. They represent a different operating model, one where parallel coordination replaces sequential handoffs as the organizing principle.
The Coordination Test
CNPV operates as an experiment in what FDA can accomplish with different incentive structures. The program functions through Commissioner discretion without statutory authorization. Its longevity depends on whether accelerated reviews maintain quality standards and whether affordability commitments produce genuine access improvements.
But whatever the program's future, it has already revealed something important about pharmaceutical operations.
The 10-12 month review period wasn't just regulatory processing. It was coordination buffer: time that masked misalignment between functions, absorbed handoff friction, and allowed sequential execution to eventually produce integrated outcomes. Companies didn't know they depended on this buffer because it was invisible, built into the system's baseline assumptions.
CNPV eliminates the buffer. And in doing so, it will expose which organizations have genuine cross-functional coordination and which have been borrowing time they didn't know they had.
Seventy-four percent of Complete Response Letters cite manufacturing and quality issues. That statistic predates CNPV. The program didn't create the coordination problem. It removed the buffer that made the problem tolerable.
The question isn't whether your organization can earn a voucher. It's whether you're ready to use one.
Because in the CNPV era, speed without coordination isn't an advantage. It's an exposure.
The organizations that will thrive aren't waiting to find out. They're building parallel coordination now, not because CNPV demands it, but because patients deserve it.
References
- U.S. Food and Drug Administration. "FDA Launches Commissioner's National Priority Voucher Program." FDA News Release, March 2025. fda.gov
- U.S. Food and Drug Administration. "FDA Awards Commissioner's National Priority Vouchers to Two Additional Merck Drugs." FDA News Release, December 19, 2025. fda.gov
- Jones Day. "Commissioner's National Priority Voucher Program and Coverage Considerations." Jones Day Insights, December 2025. jonesday.com
- BioPharm International. "CMC Issues Continue to Lead Complete Response Letters." Industry Analysis, 2024. biopharminternational.com
- Tufts Center for the Study of Drug Development. "Cost of Developing a New Drug." CSDD Impact Reports, 2024. csdd.tufts.edu
- Gilead Sciences. "Gilead Announces FDA Accelerated Approval of Livdelzi." Gilead Press Release, August 2024.
Further Reading
From Unipr Insights:
- Four Companies Wearing One Logo: The Architecture of Disconnection — Why pharmaceutical organizations operate as four separate companies sharing a single logo, and what that means for coordination
- Why Connecting Data Isn't Enough: The Coordination Imperative — The difference between connected systems and coordinated operations
- Same Day Launch: Future Blockbusters Needn't Leave Money on the Table — What enables launches within hours of approval, and why the 63-day gap is a choice
- Global Launch Delays: Impact on Patients & Revenue — The human and financial cost of coordination gaps across global markets
- Reflections on JPM 2025: The Era of Operational Excellence — Why operating margins have become the defining metric for pharmaceutical performance
Latest articles
Compliance
Unipr is built on trust, privacy, and enterprise-grade compliance. We never train our models on your data.






Start Building Today
Log in or create a free account to scope, build, map, compare, and enrich your projects with Planner.




